NOT-CLOSED PERPETUUM MOBILE ? : REVERSED ELECTRO DIALYSES IN COUNTER FLOW.
If you dilute salt water with fresh water of the same temperature that diluted salt water becomes cooler (dilution energy). In that case there is an energy source so that Reversed Electro Dialyses can work in countercurrent as 'perpetual mobile'. In any case, sodium and chlorine ions have no memory in the subsequent RED stack.
The law of conservation of energy (you cann't produce energy from nothing) prevents continuous movement that could also generate energy. There is always something, besides the law of conservation of energy, that makes the dream for this eternal motion impossible. Such as the idea of letting water rise through capillary action, which would then drip out of the capillary tubes at a higher level and thus, on a large scale, could power a hydroelectric power station. Unfortunately, the same capillary action that causes the water droplet to rise into the capillary tubes also prevents the water droplet from falling out of that capillary tube. My textbook example was a wheel that stands upright in a water container with waterproof rubber bags attached to it containing a steel marble. On one side of the wheel the steel marbles fall into a container, but on the other side they fall into the rubber bag, the volume of which increases due to the weight of the marble and the gravity force. The total volume is therefore larger on one side of the wheel and the wheel should therefore start to rotate as a result of Archimedes' law. Unfortunately, this extra upward force only compensates for the weight of the loose steel marbles. Etc.
However, I don't think the law of conservation of energy necessarily means that there can be no eternal motion. That's when a system continuously extracts heat from its surroundings. And indeed. An example of this is the 'drinking bird' which moves thanks to evaporation and therefore ambient heat. Unfortunately, this invention is unable to convert that ambient heat into really usable energy. If you think you have invented a perpetual motion machine, there must be an effect that makes your dreamed energy extraction impossible. Unless there is indeed absorption of ambient heat. Extraction of (low-calorie) ambient heat also occurs when, for example, table salt is dissolved in water or even when the solution of table salt is further diluted. Reversed Electro Dialysis works on the energy resulting from the difference between salt levels. Because the charged sodium and chlorine ions pass through ion-selective membranes to the fresh water, a voltage difference is created across these membranes and the salt water is therefore diluted.
By mixing saltwater and freshwater, blue energy (salanity gradient power) is released. The temperature then rises slightly. But after decades of desalination of seawater (Electro Dialysis) by applying a voltage difference to ion-selective membranes, research is also being conducted (by the company RED-stack on the Afsluitdijk in The Netherlands) to membranes just to generate electricity (Reversed Electro Dialysis). The waste product is brackish water. Exactly the same if you let the river water flow directly into the sea. In all schemes I encounter for mixing salt and fresh water by Reversed Electro Dialysis, the input salt water and the input fresh water come out of the RED stack as brackish water. With the salinity of both the original saline and the original fresh outflow being exactly the average of the input saline and the fresh inflow. It is therefore apparently not possible to collect the original salty input as fresh water and the original sweet input as salty water (just as in a heat exchanger the cold water entering countercurrent to the warm water becomes warmer than the average of the cold and warm input). ). And therefore, with that automatically regenerated fresh and salty water, the RED process could be repeated again to generate additional electricity with the same originally salty and originally fresh water. It would be completely contrary to the law of conservation of energy anyway.
But unfortunately so. Also with Reversed Electro Dialysis in countercurrent, the salty input and (therefore) also the sweet input does not become sweeter or saltier than the average by flowing past the sweet or salty input. So when leaving the stack, the salty input flows as brackish water in countercurrent along pure fresh water, but nevertheless no extra sodium or chlorine ion will pass through the ion-selective membrane to the then still pure fresh water, causing the input salty and the imported fresh water would become less salty or less sweet than if you were to combine that salty and fresh water directly. What is the explanation for this?
The driving force behind Reversed Electro Dialysis is diffusion. So a gas or a solution in a liquid wants to spread. In osmosis, where the spread of the dissolved molecules is hindered by a membrane, the spread of the dissolved molecules is still achieved because the water is sucked through the membrane to the solution with the highest concentration. With Reversed Electro Dialysis, this spreading of the solution is achieved by the positively and negatively charged ions of the NaCl moving through ion-selective membranes. If the salty and fresh input move between the ion-selective membranes in opposite directions, the salty input would therefore pass through pure freshwater before leaving the ion-selective membranes and should therefore become almost completely fresh, just as with the operation of a heat exchanger. And the fresh import is almost salty. As mentioned, the driving force behind RED is diffusion. So is it possible that if the salty input were to become completely fresh when fresh water passed through ion-selective membranes, the sodium and chlorine ions in the even richer part would move faster than the flow speed between the membranes than through the (ion-selective) membranes?
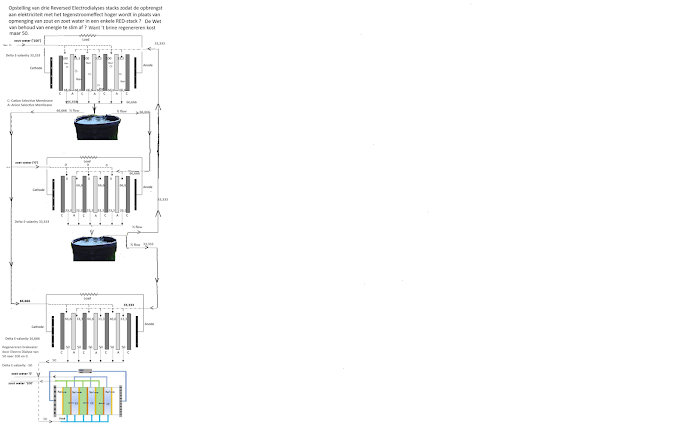
But ... if diffusion in the channels between the ion-selective membranes is the cause of the countercurrent effect in a RED stack not being successful (not to mention that it would be possible to demonstrate this), then this diffusion can still be circumvented. Namely by running the salty and sweet input in countercurrent steps through several stacks. This is possible with three regular stacks (of which the 'salty' and 'sweet' discharge is not collected separately).
1. In the first stack, the salt water ('100') is introduced as salty input. The fresh input of this stack is not the fresh water, but the 'brackish' discharge from the second stack ('33,333').
2. In the second stack, the fresh water ('0') is entered as fresh input. The 'brackish' discharge from the first stack ('66,666') is the saline input.
3. The discharge from the second stack (the one with the fresh water '0' and the brackish salt discharge from the first stack '66,666' will have a lower salinity '33,333' than the average (50) of the fresh salt and fresh sweet input.
4. The discharge from the first stack (the one with the salty water '100' and the brackish-sweet discharge from the second stack '33,333') will have a higher salinity ('66,666') than the average (50) of the fresh and salty input.
5. Because the discharge from the first stack (66,666) is saltier than the average of the salty and fresh supply and the discharge from the second stack (33,333) is sweeter than the average (50) of the salty '100' and fresh supply '0', these brackish 'salt' and brackish 'fresh' charge it over a third RED stack to generate even more electricity.
6. Because the salty supply and the other supply (the brackish 'fresh') from the first stack come together in to the same container, the amount of brackish salt discharge becomes double the supply of influent salty water. The same applies to the amount of discharge from the second stack (i.e. the brackish-fresh process water). That is why half of the flow from both the brackish-salt discharge and the brackish-fresh discharge goes to the other stack as a supply. The other half of these two containers goes to the third RED stack as brackish-fresh and brackish-salty supplies. This water coming from the third stack then becomes really brackish.
7. The salinity of the discharges (and therefore inputs) of the first two stacks (and therefore also the input of the third stack) can be calculated. To this end I set the fresh salt supply of the first stack to '100'. And the fresh supply of the second stack at '0'. I call the brackish-fresh supply of the first stack 'a' and the brackish-salty supply of the second stack 'b'. These, a and b, must together be 100. So a+b = 100. The ((brackish salt) discharge of the first stack (b) must be the average of the salt supply '100' and the brackish fresh input (a). So b = (100 +a)/2 And the ((brackish-fresh)) discharge from the second stack (a) must be the average of the fresh sweet supply '0' and the brackish-salty input (b). So a = (0+b)/2 or a= b /2
So
a + b = 100
a = b/2 or 2a = b
a + 2a = 100 3a = 100 a = 100/3 = 33.3333
b = 100 -a = 100 - 33.3333 = 66.6666.
8. Because the effluents from the first two stacks are coming together in one container and then pumped over the other stack, these stacks behave like seperated Reversed Electro Dialysis stacks without any history. In this case the arrangement is countercurrent. After all, sodium and chlorine ions have no memory.
9. In the first two stacks, the water imported as freshwater becomes process water saltier than the average of the salty and fresh input (namely 66.666 instead of 50) and the water imported as saltwater becomes process water fresher than the average (namely 33.3333 instead of 50). I believe that those extra anion and cation transport takes place through the ion-selective membranes and therefore extra electricity (energy) is generated.
10. In theory, you could not only gain extra electricity with this setup. But by using Electro Dialysis of the brackish drain of the third stack (50) even more electricity can be generated (33,333 + 33,333 + 16,666) than is needed to regenerate the brackish drain back to the influent as salt water and fresh water.
That would be completely contrary to the law of conservation of energy. So where is my fallacy? Or I'm making another mistake. Namely that the required extra energy would be released because the salt content of the water imported as salt water falls below the average of the salty and sweet input, because the dissolution of NaCl is an endothermic process and therefore also the dilution of a table salt solution.
Leon Nelen.
Former Operator Waste Water Treatment (job title inflation for sewer drain emptier)




Reacties
Een reactie posten